Severe flu risk as immune cells swap with age
Lung infections with the influenza virus or a coronavirus more frequently result in severe disease progression in older people. This is due to an excessive inflammatory reaction that causes damage to the lung tissue. The exact causes are not yet clear. Macrophages, also known as the immune system’s scavenger cells, play a role. They release pro-inflammatory messengers, which then trigger a severe immune response.
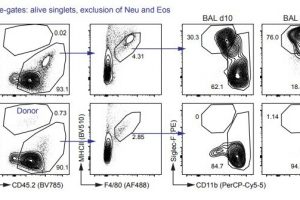
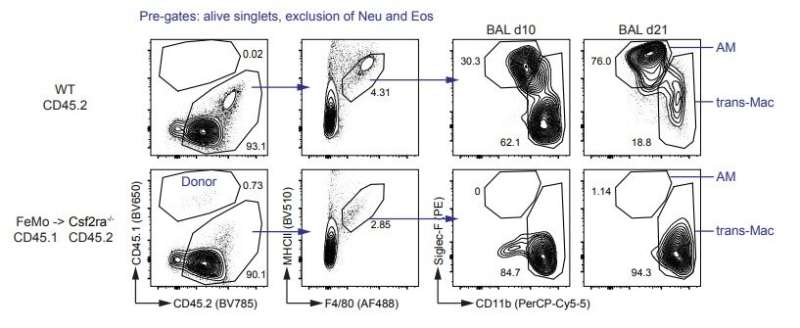
A team of researchers lead by Manfred Kopf, an immunologist and Professor of Molecular Biomedicine at ETH Zurich, has shown that in mice originally resident scavenger cells in the lung—called embryonic alveolar macrophages—rapidly die when the animals are infected with the influenza virus and are replaced after a few days by macrophages from the bone marrow. The more severe the infection, the more embryonic alveolar macrophages die. The ETH immunologists recently reported their findings in the journal Science Immunology.
Differences in function and disease progression
Macrophages have several functions, including first line of defense against pathogens. In terms of developmental biology, they can roughly be divided into two groups. In humans and in mice, all organs and tissues have resident long-lasting macrophages that are already formed during embryonic development in the fetal liver and perform specific functions in the different types of tissue. The embryonic macrophages in the lungs are involved in the vital oxygen-carbon exchange that takes place in the alveoli.
The second group of macrophages emerge during blood formation in the bone marrow and migrate through the bloodstream into different tissues, where they then mature into tissue-specific macrophages.
“Until now, it was thought that bone marrow-derived macrophages had the same function as alveolar macrophages originating in the fetal liver, and that this was true for all tissues,” explains Federica Piattini and Fengqi Li, who are part of Kopf’s research group and both first authors of the paper. They have now refuted this hypothesis, showing that, in fact, bone marrow-derived macrophages cause damage in cases of viral pulmonary infection.
The two researchers created two groups of mice: one group had alveolar macrophages originating during the embryonic stage, and the other group had bone marrow-derived macrophages. When they were infected with the influenza virus, the mice with the bone marrow-derived alveolar macrophages became much sicker and died. One reason for the severe disease progression was that these macrophages caused a strong inflammatory reaction (cytokine storm).
The authors of the study conclude that the origin of macrophages plays a critical role in the progression of the disease. And they showed that influenza infections in mice stimulate the replacement of alveolar macrophages.
Evidence of similar processes with COVID-19
According to the researchers, it makes sense and is valid to compare their influenza experiments on mice with coronavirus infections in humans. “It appears that similar processes occur in the case of COVID-19,” says Kopf. One earlier study reported that the embryonic pulmonary macrophages are largely preserved in COVID-19 patients with a mild disease progression, whereas severe disease progression is linked to inflammatory alveolar macrophages originating in the bone marrow.
However, it appeared that the embryonic alveolar macrophages were not replaced exclusively in cases of severe viral infection. Kopf’s group showed that this replacement also occurs in aging mice, even if they had never been previously infected. The transplantation of alveolar macrophages from old to young mice resulted a severe flu progression ending in death.
It is known that there is a much greater risk of severe influenza or COVID-19 infection in older people. “There are certainly several reasons for this,” says Kopf. The new data, however, suggests that the loss of embryonic macrophages over the course of life plays a role here.
Source: Read Full Article




