Potential means of improving learning and memory in people with mental illnesses

More than a dozen drugs are known to treat symptoms such as hallucinations, erratic behaviors, disordered thinking and emotional extremes associated with schizophrenia, bipolar disorder and other severe mental illnesses. But, drug treatments specifically able to target the learning, memory and concentration problems that may accompany such disorders remain elusive.
In an effort to find such treatments, Johns Hopkins Medicine researchers report they have identified a genetic variation in the brain tissue of a subset of deceased people—some with typical mental health and some with schizophrenia or other psychoses—that may influence cognition and IQ. In the process, they unearthed biochemical details about how the gene operates.
Results of their work, described in the Dec. 1 issue of the American Journal of Psychiatry, could advance the development of drugs that target the enzyme made by this gene, and thus improve cognition in some people with serious mental illnesses or other conditions that cause reduced capacity in learning and memory.
Typical antipsychotic medications that treat schizophrenia symptoms regulate the brain chemical dopamine, a transmitter of nerve impulses associated with the ability to feel pleasure, think and plan, which malfunctions in patients with the disorder. However, previous genetic studies have also shown that another brain chemical signal transmitter, glutamate, a so-called ‘excitatory’ chemical associated with learning and memory, plays a role as well. Another so-called neurotransmitter in this process, N-acetyl-aspartyl-glutamate (NAAG), specifically binds to a protein receptor found on brain cells that has been linked to schizophrenia, but how it impacts this disorder is unknown.
The research of clinical pharmacologist Kristin Bigos, Ph.D., assistant professor of medicine at the Johns Hopkins University School of Medicine, sought to explore more deeply the role of NAAG in cognitive impairment with the goal of eventually developing therapies for treating these learning, memory or concentration problems.
Using tissues gathered from a repository of brains from deceased donors belonging to the Lieber Institute for Brain Development, Bigos and her team measured and compared levels of certain genetic products in the brains of 175 people who had schizophrenia and the brains of 237 typical controls.
Bigos and her colleagues specifically looked at the gene that makes an enzyme known as glutamate carboxypeptidase II (GCPII), which breaks down NAAG into its component parts—NAA and glutamate. In the brains of people with schizophrenia and in the typical controls, they found that carriers of this genetic variant (having one or two copies of the gene variation) had higher levels of the genetic product that makes the GCPII enzyme.
In the gene for the enzyme, the only difference in the versions was a single letter of the genetic code, either G or A (for the nucleotide bases guanine and adenine). If people had the version of the gene with one copy of G, then the tissue at the front of their brain—the seat of cognition—had 10.8% higher levels of the enzyme than those who had the version of the gene with A, and if people had two copies, they had 21% higher levels of the enzyme.
To see if this genetic variation in GCPII controlled the levels of NAAG in the brains of living people, the researchers measured levels of NAAG in the brain using magnetic resonance spectroscopy, which uses a combination of strong magnetic field and radio waves to measure the quantity of a chemical in a tissue or organ.
In this experiment, they focused on 65 people without psychosis and 57 patients diagnosed with recent onset of psychosis, meaning many of them were likely to eventually be diagnosed with schizophrenia, at the Johns Hopkins Schizophrenia Center. Participants averaged 24 years of age, and 59% were men. About 64% of participants identified as African American, and the remaining 36% were white.
The researchers found 20% lower levels of NAAG in the left centrum semiovale—a region of the brain found deep inside the upper left side of the head—in the white participants both with and without psychosis who had two copies of the G version of the enzyme compared with other white people who had the A version.
To see if having the G or A version of the gene plays a role in cognition, the researchers tested IQ and visual memory in the healthy participants and those with psychosis, both white and African American. They found that people with the most NAAG in their brain (in the top 25%) scored 10% higher on the visual memory test than those in the bottom 25%. They also found that people with two copies of the G version of the GCPII sequence scored 10 points lower on their IQ test on average than the people with the A version of the gene, which the researchers say is a meaningful difference in IQ.
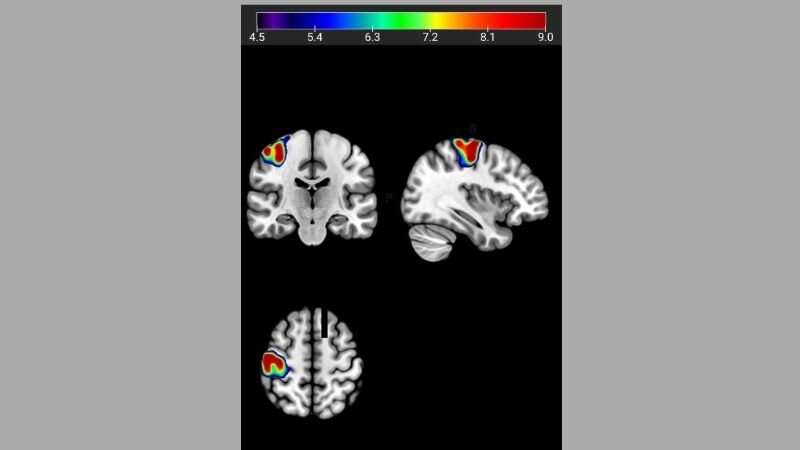
Finally, they showed that healthy carriers of the G version of the GCPII sequence had less efficient brain activity during a working memory task, as measured by functional MRI, by at least 20% compared with those people with the A version of the gene.
Source: Read Full Article




