KDM5A and PHF2 positively control expression of pro-metastatic genes

Epigenetic mechanisms present alternative targeting opportunities, but their contributions to Ewing sarcoma metastasis and disease progression remain poorly understood.
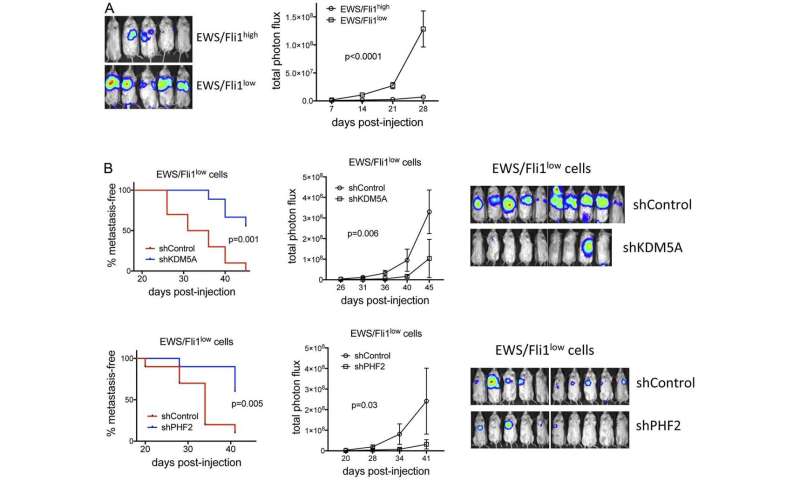
Here, the authors show that the epigenetic regulators KDM5A and PHF2 promote growth and metastatic properties in Ewing sarcoma, and, strikingly, activate expression many pro-metastatic genes repressed by EWS/Fli1. These genes include L1CAM, which is associated with adverse outcomes in Ewing sarcoma, and promotes migratory and invasive properties.
KDM5A and PHF2 retain their growth promoting effects in more metastatically potent EWS/Fli1low cells, and PHF2 promotes both invasion and L1CAM expression in this cell population.
Together, these studies identify KDM5A and PHF2 as novel disease-promoting factors, and potential new targets, in Ewing sarcoma, including the more metastatically potent EWS/Fli1low cell population.
Dr. Paul Jedlicka from The University of Colorado Denver said, “Ewing sarcoma, the second most common cancer of bone and soft tissue in children and young adults, is a biologically and clinically aggressive malignancy, with strong tendency toward metastasis and poor long-term outcomes.“
Through these and other mechanisms, EWS/Fli1 and related fusions effect dramatic alterations in gene expression, which drive aberrant cell proliferation and survival, and are necessary for tumorigenesis.
While the role of EWS/Fli1 as driver of aberrant cell proliferation, survival, oncogenic transformation and tumor growth is well established, much less is known about the mechanisms underpinning the high metastatic propensity of Ewing sarcoma.
Notably, while EWS/Fli1 imposes positive regulatory control over some pro-metastatic genes and pathways, multiple studies indicate that, on balance, EWS/Fli1 exerts a repressive effect on important metastatic properties in Ewing sarcoma.
Namely, EWS/Fli1: inhibits cell adhesion, motility and invasion in vitro; represses the expression of many metastasis-promoting genes; and inhibits organ colonization and metastasis development in tail vein injection models in vivo.
Interestingly, recent studies demonstrate that EWS/Fli1 expression is quantitatively heterogeneous within both patient-derived cell lines and tumors, with some cells expressing high EWS/Fli1 levels, and some expressing low levels.
Source: Read Full Article




