Innovative method identifies rare brain cell types for the first time

Tracking rare cell types in the brain has proved elusive. And yet alterations in some of these cells may be associated with a variety of diseases, including Alzheimer’s. Being able to find and study them could potentially open up a new world of brain analysis and disease intervention.
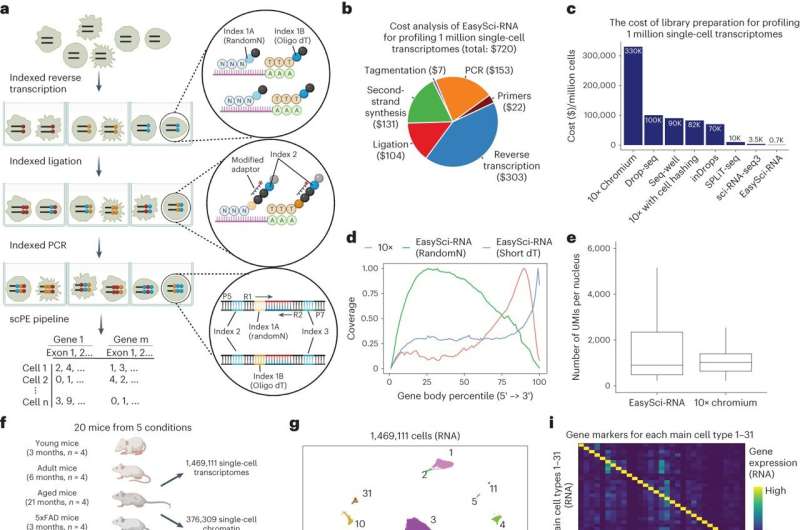
As described in a new paper in Nature Genetics, Rockefeller geneticist Junyue Cao and his colleagues have developed a low-cost, high-throughput technique for finding these secretive cells by scanning an entire mouse brain at once—a digital bucket that captured 1.5 million cells and can hold many more.
A type of single-cell sequencing called EasySci, their method can simultaneously reveal the identity of every cell entered into the system. The researchers used it to illuminate cell populations and dynamics specific to different ages, as well as to Alzheimer’s disease, in both mouse and human brains. Some cellular subtypes have never been seen before.
“A key feature of EasySci is that instead of focusing on specific brain regions, we can scan each cell, one by one, across millions of cells, to create a holistic view of the entire brain—and to identify detailed changes for different cell types,” says Cao, head of the Laboratory of Single-Cell Genomics and Population Dynamics at Rockefeller. “We can use this to understand aging and Alzheimer’s disease.”
It also reduces the cost of a million single-cell transcriptomes from $10,000 to $700—and the time necessary down to about a day.
A precious resource
When it comes to understanding neurological conditions, access to human brain tissue is essential. But because it’s such a precious, finite resource, most analyses of brain tissues rely on small sections from select areas of the brain. And yet the human brain is a fantastically complex network made up of more than 3,000 types of cells. Being able to see its molecular totality could reveal hidden cells that though rare might produce an outsized effect.
Recent advances in single-cell sequencing—a method of genetic analysis that homes in on the genetic expression and molecular dynamics of individual cells—are making it increasingly possible to find rare cells and explore cell dynamics. And while it’s still not possible to scan an entire human brain using single-cell sequencing, the mouse brain provides a good model for testing the technological potential.
For the current study, led by Cao lab researchers Andras Sziraki, Ziyu Lu, and Jasper Lee, the team used a method called combinatorial indexing to reveal more about these cells and their dynamics. The technique involves attaching ID tags to different molecules, resulting in every molecule having a unique barcode. In this way, they can then identify and tally the number of all the different cell types.
From most common to rarest
The researchers studied six types of brains: young, adult, and aged mouse brains; mouse brains with neural degeneration that mimics Alzheimer’s disease; and tissues from human brains that were either normal or had Alzheimer’s disease. The human samples came from the hippocampus, associated with learning and memory, and the superior and middle temporal gyrus, both located in the temporal lobe and linked to the processing of language and sound.
Scanning more than 1.5 million cells, the researchers identified 31 cell types and 359 subtypes in the mouse brain; nearly one-third of these subtypes had never been reported before. They discovered that the star-shaped astrocyte cells in one brain region, for example, were different from astrocytes in a different area; some subtypes even within the same region showed variations.
The most common cells were cerebellum granule neurons (32.5%), which pass information from the central nervous system to the cerebellar cortex. Rarest were inferior olivary nucleus neurons (0.05%), which are located in the brainstem and process data about the body’s movements.
“If we had only scanned 100 or even 1,000 cells, we never would have seen some of these rare cell types,” Cao says. “We needed to scan millions of cells.”
Another rare cell type they identified was the pinealocyte, a pineal gland cell that secretes the hormone melatonin, which is linked to circadian rhythm. There were just 21 pinealocyte cells in that batch of a million.
They also learned that the olfactory bulbs of young mice brim with a special group of neurons and astrocytes that more than double across the early developmental stages—not surprising for an animal that relies on a highly developed sense of smell to navigate its environment. And they found that “neurons in the olfactory bulb have many different subtypes in the same location with different functions,” Cao says.
The Alzheimer’s brain
Distinct cells—and changes—marked the Alzheimer’s brains, both mouse and human. A rare subtype of choroid plexus epithelial cells, key structural components of the blood-brain barrier that secrete cerebrospinal fluid, had been lost twice as much in Alzheimer’s brains. These cells are associated with mitochondrial genes that protect against neurodegeneration and Tau proteins in cerebrospinal fluid.
They also documented new changes in 20 cell subtypes, some of them in brain regions where they’ve never been seen before. This is important, Cao says, because many Alzheimer’s researchers focus on cortical regions or the hippocampus, which are associated with common symptoms. “But as we have shown in both human and mouse AD brains, there are many other regions that see changes,” he says.
Moreover, he adds, with conventional techniques, you can only identify two or three molecular biomarkers of disease inside each cell, but EasySci can potentially identify thousands of them. “And once you identify those ‘bugs,'” he says, “you can seek ways to correct them.”
More organs to come
In the future, Cao and his team plan to use EasySci to scan mixed tissues. “We can use this to scan many brains from diverse patients within a single experiment,” he notes. As they describe in a recent paper in Nature, they’ve already tested the approach with 101 mutant mouse embryos, scanning 1.6 million cell nuclei simultaneously.
In an upcoming study, the team shares the results of its scans of every major internal organ of the mouse at once. The mouse brain needs more attention too: the current study covered only around 2% of that total cell population, which is estimated to top out at approximately 100 million. Extending this technology to the human brain—with its staggering 170 billion cells—presents a more complex challenge and will necessitate developing new techniques.
But by focusing on increasing EasySci’s throughput power, Cao is optimistic that the method will soon be able to scan tens of millions of cells at once. “We want to further refine this method to the extent that it can be used to scan every cell, from those in the brain to the entirety of the body. This could provide critical insights for addressing cellular changes associated with aging and Alzheimer’s disease,” he says.
More information:
Andras Sziraki et al, A global view of aging and Alzheimer’s pathogenesis-associated cell population dynamics and molecular signatures in human and mouse brains, Nature Genetics (2023). DOI: 10.1038/s41588-023-01572-y
Xingfan Huang et al, Single-cell, whole-embryo phenotyping of mammalian developmental disorders, Nature (2023). DOI: 10.1038/s41586-023-06548-w
Source: Read Full Article




