New ways to prove efficacy of emerging treatments for macular diseases

Stargardt disease is an inherited eye disease. Its onset is typically in adolescence and it is among the leading causes of blindness in this age group. Symptoms range from severe loss of sharp vision to almost complete loss of sight. To date, Stargardt disease has remained untreatable.
A study now published in JAMA Ophthalmology with the participation of the University of Basel and the Institute of Molecular and Clinical Ophthalmology Basel (IOB) shows that microperimetry testing can be used to determine whether novel treatments are efficacious in slowing down vision loss.
“To develop a treatment for Stargardt disease, we first of all need sensitive examination procedures for measuring disease progression. Without these we will not be able to assess the efficacy of novel therapeutic approaches,” explains Prof. Hendrik Scholl. He is the Chair of the Department of Ophthalmology at the University of Basel, and Clinical Director of IOB. He is also the Principal Investigator for the international multicenter Progression of Atrophy Secondary to Stargardt disease (ProgStar) study.
“In the ProgStar study, we have examined structural abnormalities in the retina over time in Stargardt disease using various techniques. Structural changes are important outcome measures in interventional studies, but they must be associated with functional loss to be meaningful.”
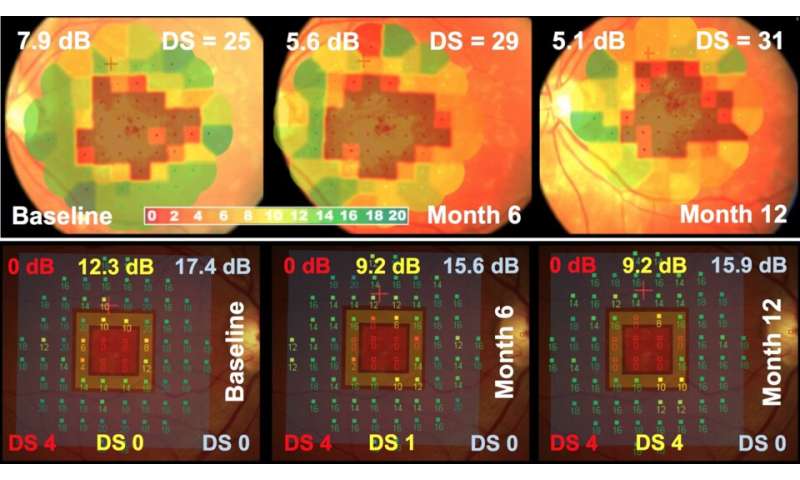
Microperimetry may be technology of choice for outcome measurements
These new findings in the ProgStar study shown that microperimetry may be the technology of choice because it allows functional deficits to be measured before they become apparent by other imaging techniques. Potentially, microperimetry may even detect changes in early stages of Stargardt disease, before structural changes have developed. This would allow interventions before the disease progresses to advanced stages,” Scholl explains.
The data presented in this study provide evidence that microperimetry is a sensitive test for detecting progression within a short study period, i.e. the yearly rate of change of macular function in patients with Stargardt disease. Microperimetry measurements therefore may serve as a useful functional outcome parameter for clinical trials investigating the efficacy of emerging treatments that aim at slowing down or stopping disease progression.
“At IOB, we are currently developing gene therapy for Stargardt disease that we plan to bring to the clinic within the next five years. We use base editing, a novel and highly promising approach, to change the mutated genetic code directly by reverting disease-causing mutations back to normal. This process is similar to correcting a single typo in a text. The results of the ProgStar study provide indispensable measurement tools for this approach. They are helping us in the development of a treatment for this blinding condition,” Scholl says.
About Stargardt disease
Stargardt disease is the most common form of inherited juvenile macular degeneration. Its onset is typically in adolescence and it is among the leading causes of blindness in juveniles. It affects the center of the retina—the macula—and leads to a progressive loss of vision in the center of the visual field. Patients can no longer see things in sharp focus, i.e. they cannot read or recognize faces and they hardly see colors. Whatever they try to see in sharp focus becomes blurred or distorted, or completely disappears.
This inherited disease is caused by genetic mutations that prevent cells in the retina from digesting the metabolic products of the photoreceptors. The toxic metabolic products accumulate and cause degeneration of the retinal pigment epithelium and photoreceptors.
Stargardt disease (STGD1; OMIM: 248200) is caused by pathogenic mutations in the ABCA4 gene. The protein product of the ABCA4 gene is responsible for removing a toxic degradation product of vitamin A that is produced during the visual process in the retina.
Source: Read Full Article




