Potential strategy for treating liver cancer: Study discovers signaling pathway mediated by extracellular vesicles
A research team led by Professor Judy Yam Wai-ping from the Department of Pathology, School of Clinical Medicine, LKS Faculty of Medicine, the University of Hong Kong (HKUMed) has made a significant breakthrough in unveiling an unrecognized signaling pathway mediated by circulating small extracellular vesicles (sEVs) derived from liver cancer patients that promotes liver cancer metastasis. This discovery presents a potential therapeutic strategy for treating liver cancer. The findings have been published in Advanced Science.
Liver cancer is the fifth most common cancer and the third leading cause of cancer death in Hong Kong. As a hypervascular tumor, liver cancer is largely driven by the modulation of tumor-derived small extracellular vesicles (sEVs) within the tumor microenvironment.
Enhanced vascularization allows tumor cells to enter the bloodstream, facilitating their dissemination to distant loci and thus cancer metastasis. Growing evidence suggests that tumor-derived sEVs play a role in modulating angiogenic signaling. Understanding the mechanisms by which sEVs regulate angiogenesis in liver cancer could lead to the development of new therapeutic strategies.
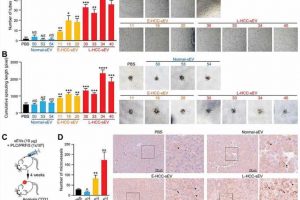
Proteomic profiling of circulating sEVs of control subjects and Hepatocellular Carcinoma (HCC) (the most common type of primary liver cancer) individuals revealed von Willibrand factor (vWF) to be upregulated progressively along the developmental stages of liver cancer. The drastic elevation of sEV-vWF level in HCC patients indicated its potentiality as a non-invasive diagnostic marker for liver cancer. The research team also showed the inducing ability of circulating sEVs of late-stage patients in liver cancer development and metastasis was dampened by anti-vWF antibody, demonstrating the crucial role of sEV-vWF in liver cancer.
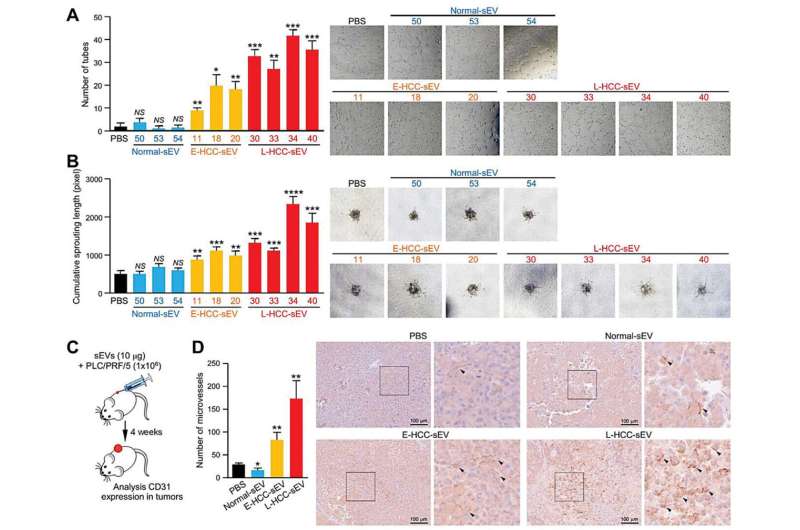
vWF-enriched sEVs derived from liver cancer cells displayed a significant promoting effect on angiogenesis, tumor-endothelial adhesion and vascular permeability. The growth factors released by the endothelial cells could in turn promote cancer cell growth and motility. The study unveils an unrecognized mutual stimulation between tumor and endothelial cells initiated by sEV-vWF. Using a mouse model of liver cancer, the team observed that the enhanced angiogenesis mediated by sEV-vWF facilitates liver cancer development and distant metastasis, which is crucial to liver cancer development and metastasis.
The team further demonstrated that the combination treatment of Sorafenib (the standard first-line treatment of advanced liver cancer patients), with anti-vWF antibody or the pan-FGFR inhibitor Erdafitinib, significantly improved the treatment outcome than Sorafenib treatment alone in patient-derived xenograft mouse model. The results suggested a novel therapeutic approach for liver cancer by blocking sEV-mediated tumor-endothelial intercellular communication.
“There is limited curative therapeutic option for cancer patients. Understanding the molecular basis of liver cancer will provide insights into new effective strategies to treat liver cancer. In this study, we identified a pivotal functional role of vWF carried by circulating sEV obtained from the advanced stage liver cancer patients in inducing neoangiogenesis.
“Our study not only discovered the role and molecular basis of vWF delivered by sEV in liver cancer but also suggested that the blockade of intercellular communication within the tumor microenvironment is a new therapeutic approach for liver cancer,” said Professor Judy Yam Wai-ping, professor, Department of Pathology, School of Clinical Medicine, HKUMed.
More information:
Samuel Wan Ki Wong et al, Small Extracellular Vesicle‐Derived vWF Induces a Positive Feedback Loop between Tumor and Endothelial Cells to Promote Angiogenesis and Metastasis in Hepatocellular Carcinoma, Advanced Science (2023). DOI: 10.1002/advs.202302677
Journal information:
Advanced Science
Source: Read Full Article




