Research highlights disrupted NAD(H) homeostasis as a potential therapeutic target to combat tuberculosis
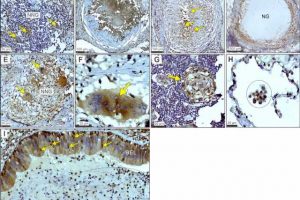
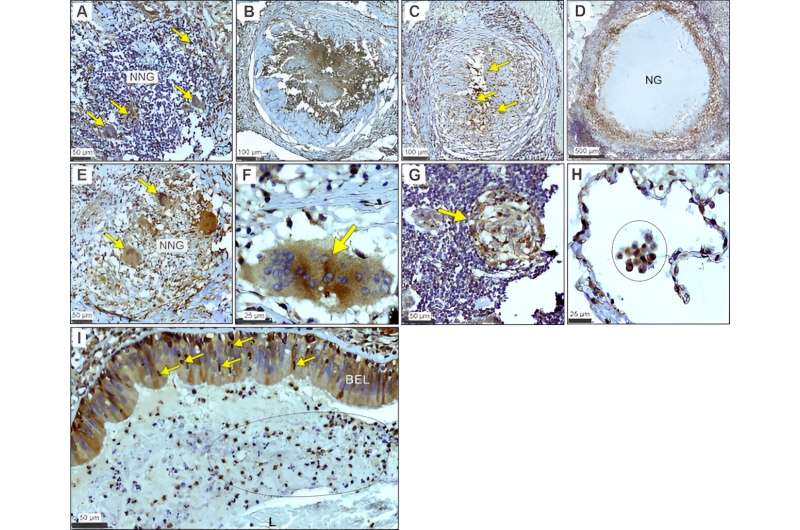
It has been uncertain how Mycobacterium tuberculosis deflects the immune response in humans, though evidence has pointed to host immunometabolism—the intrinsic link between metabolism in immune cells and their immune function. The pathogen M. tuberculosis is known to disrupt a metabolic pathway called glycolysis in infected myeloid cells, which include macrophages, through an unclear mechanism.
A more accurate understanding of this pathogenic mechanism could provide a target against the bacterium that caused 1.6 million deaths in 2021, along with 10 million new cases of tuberculosis every year.
Now a study published in Nature Communications by researchers at the University of Alabama in Birmingham and the Africa Health Research Institute (AHRI), shows how M. tuberculosis perturbs homeostasis of the high-energy molecule NADH and reprograms glycolysis in myeloid cells. This highlights glycolysis as a potential therapeutic target to combat the world’s leading infectious disease killer.
Glycolysis is the pathway that converts glucose into pyruvate while forming the high-energy molecules ATP and NADH. But the pathway can run in either direction, and researchers took advantage of that to apply a more selective approach to inhibiting glycolytic flux. Previous experiments have taken a more sledgehammer-like approach, such as using an inhibitor that blocks uptake of glucose into myeloid cells.
The reversible process of lactate fermentation is catalyzed by the enzyme lactate dehydrogenase, or LDH. LDH has four subunits, a mix of LDHA and LDHB subunits. When LDH is made mostly of LDHA subunits, a subunit that is predominantly expressed in myeloid cells, it preferentially converts pyruvate to lactate, and NADH to NAD+. However, an LDH made of LDHB subunits favors the opposite reaction.
The role of LHDA in tuberculosis pathogenesis is unknown. The UAB and AHRI researchers looked at resected lung tissue from patients with tuberculosis and found that myeloid, bronchial epithelial cells and lymphocytes stained positive for LDHA as they engaged in distinctive immunological phenomena like granuloma formation and alveolitis. “These data implicate LDHA as an important metabolic protein in the immune response in human tuberculosis lesions,” said Adrie Steyn, Ph.D., senior author of the research.
Knowing that NADH/NAD+ regulates glycolysis at defined steps, Steyn and colleagues hypothesized that NAD(H)-mediated glycolytic flux in myeloid cells protects the host against M. tuberculosis infection. To test this, they created mice that lack the LDHA subunit in myeloid cells. These cells have a reduced glycolytic capacity because the altered LDH function, composed only of LDHB subunits, decreases their ability to regenerate NAD+ from NADH in the presence of pyruvate.
The LDHA-deficient mice, when infected with a low dose of M. tuberculosis, were more susceptible to infection and had a significantly reduced survival time. Also, gross pathology and histopathology of the lungs were worse in the LDHA-deficient mice. Furthermore, wild-type mice initially mount a robust inflammatory response against the M. tuberculosis infection as a protective immune response, while the LDHA-deficient mice had a striking absence of early inflammation.
“This suggests that LDHA is necessary for protection against tuberculosis and that glycolytic flux in myeloid cells is essential for the control of M. tuberculosis infection and disease,” Steyn said.
Despite evidence of a reduced immune response, when researchers quantified gene expression in the lungs of the LDHA-deficient mice, they found that mRNAs associated with inflammatory processes were among the most enriched, especially a robust interferon-gamma gene set.
“The robust interferon-gamma gene expression signature in more susceptible mice with a blunted immune response was particularly intriguing since interferon-gamma is an indispensable antimycobacterial cytokine widely considered to be protective in tuberculosis,” Steyn said.
This conundrum was solved through bioenergetics experiments that showed mouse macrophages require LDHA and its LDH-mediated NAD+ regeneration for their metabolic response to interferon-gamma.
Since NAD+ depletion appeared to be central to the glycolytic inhibition caused by M. tuberculosis, the researchers asked whether the addition of an NAD+ precursor, nicotinamide, would alter the ability of macrophages to mount an immune response.
Nicotinamide was found to increase the glycolytic capacity of M. tuberculosis-infected bone marrow-derived macrophages. The researchers hypothesized that nicotinamide acts as a host-directed therapy by enhancing glycolysis in M. tuberculosis-infected macrophages through its conversion to NAD(H) via the NAD+ salvage pathway.
They found that nicotinamide was an effective treatment for tuberculosis in in vitro experiments where they infected macrophages with luciferase-expressing M. tuberculosis. The infected macrophages showed that nicotinamide reduced luminescence at 48 hours post-infection dose dependently, and this reduction of the pathogenic bacteria depended on glycolysis. In a mouse model, feeding the mice nicotinamide for four weeks, beginning either three days or 28 days post-infection, showed a tenfold reduction of the M. tuberculosis burden in the lungs, and it also reduced inflammation in the lungs.
Nicotinamide was first described as a treatment for tuberculosis in the 1940s, via a different mechanism; but it was largely abandoned when much more effective drugs were discovered in the golden age of antibiotics.
However, the landscape of tuberculosis has shifted dramatically in the last 60 years. The incidence of tuberculosis has increased to more than 10 million new cases annually, and the pathogen has developed resistance to the frontline drugs that displaced nicotinamide, Steyn says.
“We have provided further evidence of a host-dependent effect of nicotinamide, the metabolic requirements for its activity and a modern-day demonstration of its efficacy as a treatment for tuberculosis, using two treatment regimens in vivo,” Steyn said of the current study.
“Logistically, nicotinamide satisfies many of the criteria for an optimal novel tuberculosis treatment regimen set forth by the World Health Organization. It is inexpensive, orally bioavailable, shelf-stable, remarkably safe and tolerable, and it is well-studied and routinely used in humans for various indications. Ultimately, these characteristics make nicotinamide appealing as an old tool in a modern setting.”
An unanswered question remains, Steyn says. How does M. tuberculosis deplete NAD(H) levels? A partial explanation, the researchers say, may be the secretion by M. tuberculosis of tuberculosis necrotizing toxin, or TNT, an NAD+ glycohydrolase. This toxin, reported by UAB’s Michael Niederweis, Ph.D., in 2015, is the first toxin ever found in M. tuberculosis during 132 years of study. TNT in wildtype M. tuberculosis significantly reduces NAD+ abundance in infected macrophages.
Steyn is a UAB professor of microbiology and oversees labs at UAB and at the AHRI, in Durban, KwaZulu Natal, South Africa, an area that is the worldwide epicenter for tuberculosis infections. Niederweis is a professor in the UAB Department of Microbiology.
First author of the new study, “NAD(H) homeostasis underlies host protection mediated by glycolytic myeloid cells in tuberculosis,” is Hayden T. Pacl, M.D., Ph.D., UAB Department of Microbiology.
Co-authors with Steyn and Pacl are Krishna C. Chinta, Vineel P. Reddy, Sajid Nadeem, Ritesh R. Sevalkar and Joel N. Glasgow, UAB Department of Microbiology; Anupam Agarwal, UAB Department of Medicine, Division of Nephrology; and Kievershen Nargan, Kapongo Lumamba and Threnesan Naidoo, AHRI, University of KwaZulu Natal, Durban, South Africa. Naidoo also has an appointment at Walter Sisulu University, Eastern Cape, South Africa.
More information:
Hayden T. Pacl et al, NAD(H) homeostasis underlies host protection mediated by glycolytic myeloid cells in tuberculosis, Nature Communications (2023). DOI: 10.1038/s41467-023-40545-x
Journal information:
Nature Communications
Source: Read Full Article




