‘Seeing’ the unseen: A way to pinpoint elusive cardiac conduction tissue
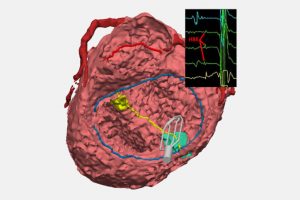
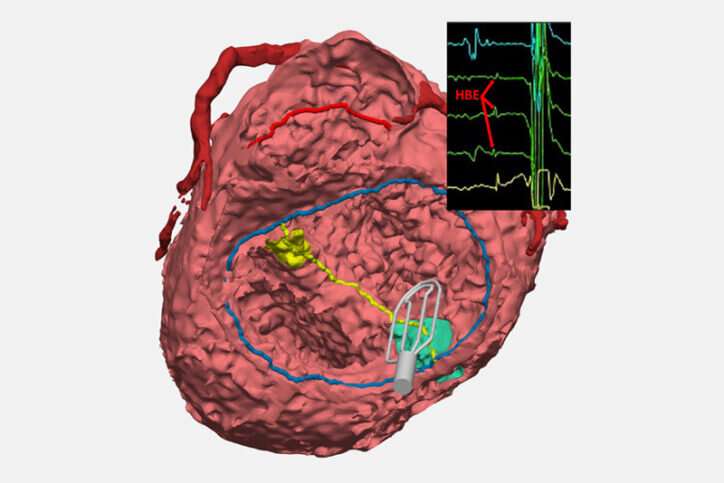
When patients with congenital heart issues have an operation, surgeons have to proceed with an “eye of faith” as they work around conduction tissue—a network of cells and electrical signals that control the beating of a heart.
Not visible to the naked eye, conduction systems vary person to person, but they’re particularly difficult to pinpoint in patients with complex congenital heart defects (CHD). When surgeons can’t locate conduction tissue during surgery, they risk accidentally injuring it. This can cause heart block, a form of arrhythmia that often creates the need for a pacemaker and more operations.
Surgeons have long wanted to “see” the conduction system. Now, a promising technique used by Boston Children’s physicians provides that vision. They believe their innovative work could lead to consistent identification of conduction tissue in complex cases of CHD.
“For certain types of CHD, it’s already been a major game-changer,” says Eric Feins, MD, a surgeon in the Benderson Family Heart Center’s Department of Cardiac Surgery. “We’re understanding where conduction tissue is—and sometimes finding out it’s in a location we didn’t expect.”
Finding a tool that kept pace with surgery
The innovation is a collaboration between surgeons from the Department of Cardiac Surgery and cardiologists from the Electrophysiology Service in the Department of Cardiology. The new technique updates an approach not often used: A catheter collects electrical signals on open, beating hearts during surgical repair. The technique lets surgeons mark the spot of conduction tissue with a marker or small suture so they can avoid injuring it.
For the two cardiologists who spearhead the effort with Feins—Elizabeth DeWitt, MD, and Edward O’Leary, MD—preventing heart block is a much-preferred alternative to implanting a pacemaker after a child has developed a postsurgical arrhythmia related to scarred conductive tissue.
“That child is pacemaker-dependent for the rest of their life,” DeWitt says. “That’s decades and decades of needing replacements for the leads that provide electrical impulses to the heart and replacements of batteries—as well as all the other complications associated with lifelong dependency on a pacemaker.”
As far back as the 1970s, clinicians attempted to use electrophysiology (EP) tools to map the conduction system during surgery, but it didn’t become a standardized practice for a variety of reasons. With cardiac surgeons performing more complex CHD operations each year, leading to an increase in heart block, DeWitt and O’Leary took another look at the tools of their trade to prevent injuring conduction tissue.
After some testing, O’Leary found that an FDA-approved grid-mapping catheter that’s often used by electrophysiologists to treat arrhythmias could quickly and accurately pinpoint conduction tissue during surgery. “It allows for very quick identification and communication, from the signals that we see on the screen to what the surgeons are seeing in the operating field,” O’Leary says.
Feins likens this EP tool to a metal detector that helps beachcombers find objects buried in sand. “It collects these signals, which the electrophysiologist reads out in real time, and that tells us where conduction tissue runs. We can then mark it on the heart so that we know when we do that part of the repair, we’re not going to injure the conduction system.”
Tool points to tissue in unexpected places
In many complex CHD cases, surgeons previously had to estimate where conduction tissue was. “In some areas, we were kind of tiptoeing along,” Feins says. Now, the EP tool allows surgeons to improve surgery in the moment and create a catalog of findings that can be further studied. For instance, they’re finding that anatomical variables, such as the position of ventricles or the heart itself, can influence the location of the conduction system.
The EP tool has aided more than 150 surgeries at Boston Children’s in the past two years and has prevented heart block in many patients—especially those with heterotaxy who need complex biventricular repair. In that patient group, heart block has decreased from over 14 percent to less than 3 percent when conduction mapping is performed during surgery.
Spreading the word about the new technique
Along with Jocelyn Davee, an engineer at the Benderson Family Heart Center, Feins, DeWitt, and O’Leary are now working on several fronts to share the insight they’ve gained from EP mapping and advance its mainstream use in complex CHD surgeries.
They’ve described their findings at several medical conferences. And the abstract for Feins’ upcoming presentation at the annual meeting of the American Association for Thoracic Surgery has already won the organization’s Presidential Abstract Recognition Award. The group is now creating a full research paper.
Meanwhile—with the help of the Computational 3D Visualization Program—Davee and fellow engineers Noah Schulz and Emily Eickhoff are creating 3D models of the heart of every CHD patient who benefits from EP mapping. Ultimately, those models will be available in a digital library so clinicians can review the influences that different anatomies have on conduction and take a more personalized approach to a surgery. The team recently won a $50,000 AATS grant to support this work.
Source: Read Full Article




